
Environmental footprint reduction is something that life sciences companies cannot afford to ignore, but it doesn’t need to be costly. In fact, innovative technology can support sustainability approaches that offer a way for businesses to reduce their overheads, enhance their reputation, and be more commercially competitive.
Desde los productos farmacéuticos hasta la tecnología médica, todas las organizaciones del sector de las ciencias de la vida operan en un panorama sometido a una gran presión. Los reguladores exigen una mayor trazabilidad y seguridad. Los clientes quieren un producto eficaz que sea fácil de usar. Los patrocinadores y los responsables políticos exigen un progreso cada vez más rápido en la eliminación de las emisiones de carbono y del desperdicio de materiales.
These may seem like contradictory forces, pulling businesses in different directions and frustrating progress. However, advancements in automation and cloud technology make it possible to address these demands in parallel through several efficient, unified solutions.
As a result, companies can not only reduce their environmental footprint, but benefit from reduced regulatory risk, higher customer satisfaction, stronger ESG (Environmental, Social, and Governance) credentials, and lower overheads at once.
Almost every RFP that we receive now contains questions about our approach to sustainability. Our customers want to know about the processes we have in place for measuring our carbon footprint, and what measures we are taking to reduce our impact on the environment. When you’re shortlisted, they’ll dig even deeper, asking specific questions about plastic use in packaging, the prevalence of single-use shipping systems, alongside all the usual questions about your buildings and employee-generated emissions.
Rhys Evans
Senior Vice President, Global Operations at RxSource
Demand for progress on sustainability within the life sciences industry is universal – and increasing. 78% of consumers want pharmaceutical companies to do more on Corporate Social Responsibility (CSR)¹. Meanwhile, industry leaders are busy setting a high watermark for the industry. AstraZeneca aims to achieve zero carbon operations by 2025 and a carbon-negative value chain by 2030 ², while Pfizer targets internal carbon neutrality by 2030 and net zero by 2040³.
It’s clear that identifying and mapping routes to effective, practical change on waste is needed for companies to lift themselves on this rising tide of sustainability – rather than being dragged under by it.
This paper explores the sustainability and waste reduction opportunities open to proactive life sciences companies. It covers:
The life sciences industry is responsible for between 4-5% of Global Greenhouse Gas (GHG) emissions and a similar proportion of toxic pollutants4, putting it among the worst offenders of the world’s service industries.
Pharmaceuticals produce 55% more GHG emissions, per dollar of revenue than the automotive sector5
Of course, some emissions are unavoidable in the delivery of life-changing products and services. However, for life sciences, the intensive production of waste is often a mechanism for reducing risk.
Take, for example, the function of:
Drug Waste
Packaging Waste
A 2022 World Health Organization report found that the global pandemic caused an additional surge in healthcare waste, mainly through demand for Personal Protective Equipment (PPE), testing kits, and vaccines6 .
In a world where further epidemiological events and health system strains are more likely due to climate change and pollution, failing to tackle sustainability issues in life sciences means taking with one hand while giving with the other. It’s key that we ensure that sustaining a larger, more resilient health system doesn’t come with an impossible environmental price tag.
There is also increasing awareness of the need to account for emissions throughout an organization’s supply chain, with carbon audits looking at scope 2 and 3 emissions that fall outside of their direct control.
Customers working on scope 3 emission strategies often approach Loftware for emissions data when considering the breadth of footprint for a software solution (e.g. energy to power servers and cool data centers). However, Cloud solutions are normally more efficient than on-premise and we’re determined to better understand how we can more effectively measure emissions, so that we can continually reduce them or mitigate their impact.
Senior Account Executive Life Sciences EMEA, Loftware
As we’ve seen, systemic waste has a function in reducing compliance risk and focusing material loss in the desired areas (such as packaging rather than product). However, evidence suggests that these environmentally costly methods may not be fit for purpose. In 2022 John Blake, a Senior Research Director at Gartner delivered a presentation at Loftware’s Convergence conference, highlighting that the average cost of non-compliance in the pharmaceutical industry has grown by 43% over the past nine years to exceed $50B annually.
Non-declared allergens, mislabeling, and inaccurate packaging graphics are the leading causes of product delays and recalls, causing millions in losses for manufacturers and huge volumes of waste.
While there are issues with existing methods, movement toward sustainable techniques doesn’t match up to aspirations. According to life sciences organization, Owen Mumford, “packaging is another area where 76% of pharmaceutical companies have a sustainability policy, but only 13% of companies in our review had translated policy into actual targets”7.
Ultimately, new solutions are encountering an all too familiar foe - financing. While larger companies can absorb the costs of reusable packaging or switch to renewable energy generation, this often isn’t an option for smaller companies living for the next round of funding.
To drive sustainable shifts across the sector at pace, it’s clear that waste reduction solutions need to offer savings and business efficiencies, rather than high up-front costs to simply achieve compliance.
Life sciences companies looking to eliminate waste from their manufacturing and supply chain need to be aware of roadblocks in several key areas.
Multi-regional or multi-national markets create complexity in labeling, packaging, and distribution. The risk of errors and recalls increases as companies work across multiple regulatory landscapes, each with differing languages and labeling conventions.
Delivery across jurisdictions and large distances also poses a challenge for sustainability innovations. Reusable delivery systems, like water-filled biodegradable packaging, offer outstanding reuse potential – RxSource now uses this medium for approximately 95% of the shipments from their facility in Ireland.
However, reusable delivery systems don’t yet offer thermal stability for the same durations as polystyrene – with a typical source-to-terminal time limit of 48 hours, meaning they’re not practical for long-haul deliveries given the potential for customs delays.
The prevalence of couriers offering sustainably fueled transport options, as well as options like pickup and return for reusable shipping systems, is increasing. Despite this, higher costs, route limitations, and additional logistical burdens (particularly for reusable shippers) stop them from being the default choice yet.
Switching to these new shipping systems may also reduce the range of couriers and packaging manufacturers available, presenting strategic risks. Not only that, but multi-use shipping systems may demand adequate on-site storage and more deliveries to your packaging sites – so joined-up planning to align Enterprise Resource Planning (ERP) and Warehouse Management (WHM) systems is essential. By integrating and extending labeling solutions as part of this strategy, suppliers can decrease the need for relabeling, reducing the waste it causes.
Major legislative changes continue to roll in, with traceability emerging as a major focus. In 2023, the European Commission proposed the largest reforms of EU general pharmaceutical legislation since the mid-2000s8 , which includes a push on environmental sustainability. At the same time, serialization enhancements set out by the FDA in 2023’s Drug Quality and Sustainability Act (DQSA) are forcing manufacturers to integrate new processes and equipment9 .
While e-labeling solutions offer opportunities to collect and store more information, supporting consumer engagement and anti-counterfeiting measures, any adjustments to labeling and certification are challenging within the existing regulatory framework for life sciences businesses, and further complicated by the current lack of agreement on industry best practices.
Personalized and precision clinical trials are emerging as a new class of study, powered by advancements in genetic and molecular profiling. Treatments are adapted in real-time to optimize drug use and patient outcomes, requiring dependable and instant access to variable drug supply.
Expansive on-site drug stocks lead to costly waste. This is even more pronounced with the rise of biologics, which offer exciting new medical possibilities but can range up to $500,000 for a year’s course of treatment10. Furthermore, they often require energy-intensive cold storage facilities for transportation and storage.
A shift to a ‘Just In Time’ (JIT) approach is a sustainable way to accommodate flexible treatment regimens, but robust systems are required to ensure that trial packs will arrive at clinical sites as needed. This remains an area of massive untapped potential for many life sciences businesses.
Digital packaging is poised to explode across industries like e-commerce, furniture, and fashion, bringing major benefits in terms of both transparency and the user experience.
Digital integrations like QR codes already offer potential attractive improvements in life sciences, boosting the volume of information provided to patients, with new personalization and translation options, and offering a route to accelerate product recalls when necessary. Automatic e-label updates could allow for improvements like responsive expiry date adjustment and advisory updates.
Despite the apparent benefits, regulatory, safety, and accessibility concerns make it an uncertain prospect. Making smart devices essential could risk alienating certain demographics or prevent patient access to key information if away from devices. On the supply side, server connectivity and IT system reliability pose new risks to the availability and update of key label data such as expiry dates.
While digital packaging will make some headway in life sciences, there’s little prospect of eliminating physical labeling and associated waste in the near to medium future given these concerns and how they relate to patient safety.
Adopting a cloud-first approach is an excellent choice for businesses at every level because of its potential to deliver process efficiencies. These efficiencies mean lower (human, material, energy) resource use – reducing overheads and emissions in tandem.
By uniting environmental performance and financial performance in this way, the cloud delivers clear benefits at any stage of business maturity.
Consider some typical results of adopting a cloud labeling solution within a life sciences business:
A cloud-based labeling solution doesn’t just increase environmental and economic performance – it provides a ‘single source of truth’ that runs through the core of your business operations.
Take labeling and artwork management in pharmaceuticals as an example:
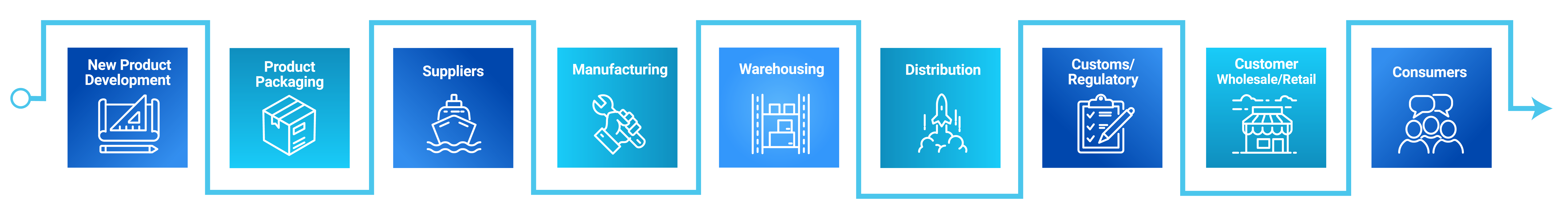
Labeling touches all corners of an organization, acting as the vehicle for data that is critical at every step of the value chain – serial numbers, origin certificates, batch information, ingredients, and dosage data, to name a few.
Moving this into the cloud positively impacts the entire enterprise, from manufacturing and distribution, right up to end users. Establishing a single source of truth across a business makes labeling part of a larger whole, with the potential for close integration into wider ERP, WMS, or Product Lifecycle Management (PLM) systems.
Many operators still rely on fragmented labeling systems which vary between warehouses, facilities, or regions. Non-universal updates, absent templates, or recurrent human error guarantee perennial issues.
By offering a centralized cloud-based labeling solution that provides instant access to label templates throughout a business or across multiple sites, Loftware helps companies improve consistency and accuracy, while also reducing the likelihood of mislabeling. As a result, there are far fewer instances where products need to be withdrawn, recalled from the market, and scrapped as waste. This in turn reduces a company’s environmental footprint, empowering them to meet their sustainability goals.
SVP of Marketing and Product Management¹¹, Loftware
By moving to a cloud-based labeling solution, life sciences businesses can:
A JIT approach to clinical trials allows for excess drug waste to be drastically reduced by matching supply to patient needs on a real-time basis. Trial packs are only dispatched to clinical sites when patients are recruited.
Cloud labeling solutions mitigate the risks associated with this approach, providing responsiveness and accuracy that are critical to success, and can facilitate automation. Without robust systems in place, trial sponsors might be tempted to accept added costs and wastage through conventional approaches, rather than risk delays or participant loss.
On average, each day of delay in a clinical trial has a direct cost of $37,000 — as well as a further $600,000 to $8m in lost opportunities12.
Clinical and procurement teams can also enjoy shared access to supply chain information through a ‘single source of truth’ on the cloud, bringing them out of their traditional siloes and making collaboration easier.
A JIT system results in more frequent, smaller deliveries, potentially resulting in more packaging waste and transport emissions. However, the financial and environmental costs of these are often more than offset by the reduction in wasted Investigational Medicinal Products (IMPs), comparators, and ancillaries. Financial savings from reduced overage could be reinvested in sustainable packaging and fleet options, to further reduce total process waste.
JIT is also a critical component in the successful delivery of efficient decentralized digital clinical trials which have the potential to reduce greenhouse gas emissions by 90% compared to conventional trial models13.
As with many industries, companies in the life sciences sector are under pressure to deliver greater oversight of their supply chain from end-to-end. Adopting cloud-based labeling makes it easier to provide a full audit trail of a product – from the raw material stage through to manufacturing and distribution, before putting it in the hands of the end user.
By connecting stakeholders across the entire supply chain through cloud platform services, you can create a verifiable ‘single source of truth’. As a result, effective governance, certification, data sharing, and standardization can go from fiendishly difficult to rapidly achievable.
Cloud-enabled companies are ideally placed to gain or retain beneficial labels like the EU Ecolabel14, which only certifies products after verification of the total product lifecycle from extraction to disposal.
By using cloud-based paper trails to futureproof against environmental regulations, such as France’s anti-waste for a circular economy law15, businesses can grow with clear horizons rather than scrambling to comply.
Our purpose is to improve the lives of patients globally, which is why setting meaningful targets to reduce our environmental impact is critical, to build a sustainable future for patients and the sector.
James Burt
James Burt, Pharmonovia CEO¹⁶
Adopting cloud solutions can offer sustainability benefits across the entire value chain – from reducing direct waste because of everyday activities to unlocking greener methods for labeling or distribution.
Placing waste reduction at the core of your business activities doesn’t just help the environment. It directly improves the experience of your staff, your stakeholders, and end users – contributing to health and reputation as an effective, purpose-led life sciences business.
30-90% reduction in corporate GHG emissions by switching to cloud computing, according to a study by microsoft, Acenture & WSP — with smaller businesses enjoying the biggest % decreases17.
Wasted resources always come with a cost – to both the planet and the bottom line. At a time when the next generation of pharmaceuticals is coming in the form of high-cost biologics, narrow shelf lives, and personalized medicine, it’s no longer feasible to accept typical levels of drug waste.
It is estimated that, in a typical trial, for every 700 doses of a medicine distributed, around 500 are never used – a wastage rate of around 60 to 70 percent.
Clinical Trial Sales Director at Loftware
Cost savings enabled by global labeling solutions aren’t just gained through efficiency. It’s estimated that production line shutdowns caused by misprinting in clinical trials, for example, add up to an annual average of $1.3M for businesses worldwide.
Further cost savings are found at every stage of the value chain, in both directions. Considering additional benefits like reduced overheads, fewer non-compliance fines, and lower recall costs, amongst others, cloud solutions are a powerful course of treatment for any life sciences business.
It is beneficial for pharmaceuticals and life sciences companies to take a step towards sustainability: Firms that pursue a sustainable strategy and solidify their reputation as a sustainable company will gain the trust of governments, global institutions, and other stakeholders – partners on whom the long-term success of the industry depends.
Dr. Robert Paffen
Partner, Risk Consulting Leader PwC Europe, PwC Deutschland ¹⁸
Reducing waste as a life sciences business has a wide and expanding range of benefits, for the organization, the stakeholders, and the natural world.
Sustainability measures are no longer a cost to be borne for reputational or regulatory reasons. They’re an opportunity to streamline, grow, and become more competitive. A pivotal shift is occurring across the life sciences industry, in which it’s becoming clear that resource efficiency through digital centralization is central to continued success – both as a business and as a provider of life-changing medical products and services.
